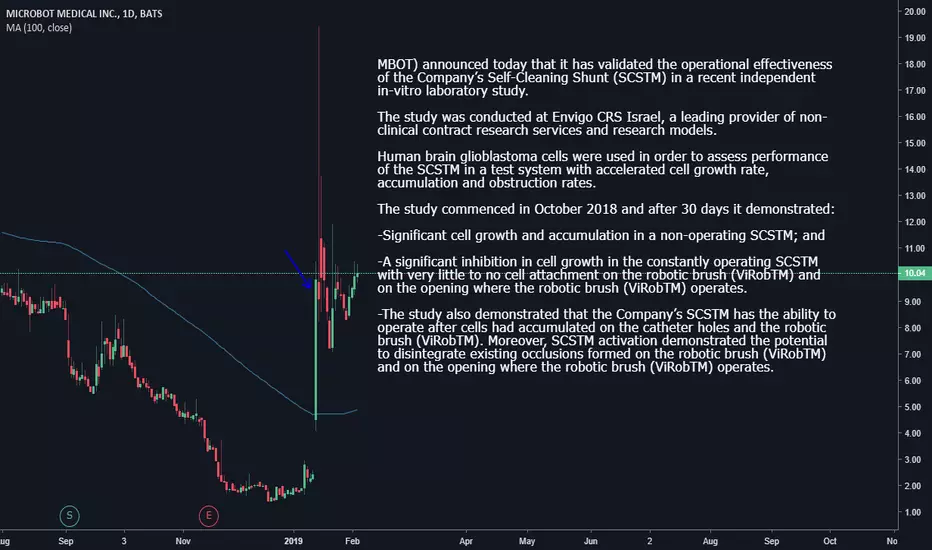
In addition to this study, the Company previously announced data from two pre-clinical studies that were performed at leading U.S. academic institutions:
-In-vitro study, which was performed at Wayne State University, supports the SCSTM’s potential as a viable technology for preventing occlusion in shunts used to treat hydrocephalus.
-In-vivo animal study, which was performed at Washington University School of Medicine in St. Louis, supports the safety profile of the Company’s SCSTM as a CSF catheter.
The primary and secondary endpoints will seek to validate the safety and efficacy of the SCSTM that will be activated in both in-vitro (lab) and in-vivo (animal) models.
The Company’s objective is to conclude the follow up study and announce the data in the second half of 2019.
The Company plans to use the findings either for its regulatory submissions in the US, Europe and other jurisdictions, or as part of a pre-submission meeting request, depending on the final results of the ongoing follow-up study.
microbotmedical.com/investors/press-releases-and-news/?pressid=2383192
-In-vitro study, which was performed at Wayne State University, supports the SCSTM’s potential as a viable technology for preventing occlusion in shunts used to treat hydrocephalus.
-In-vivo animal study, which was performed at Washington University School of Medicine in St. Louis, supports the safety profile of the Company’s SCSTM as a CSF catheter.
The primary and secondary endpoints will seek to validate the safety and efficacy of the SCSTM that will be activated in both in-vitro (lab) and in-vivo (animal) models.
The Company’s objective is to conclude the follow up study and announce the data in the second half of 2019.
The Company plans to use the findings either for its regulatory submissions in the US, Europe and other jurisdictions, or as part of a pre-submission meeting request, depending on the final results of the ongoing follow-up study.
microbotmedical.com/investors/press-releases-and-news/?pressid=2383192
免责声明
这些信息和出版物并不意味着也不构成TradingView提供或认可的金融、投资、交易或其它类型的建议或背书。请在使用条款阅读更多信息。
免责声明
这些信息和出版物并不意味着也不构成TradingView提供或认可的金融、投资、交易或其它类型的建议或背书。请在使用条款阅读更多信息。