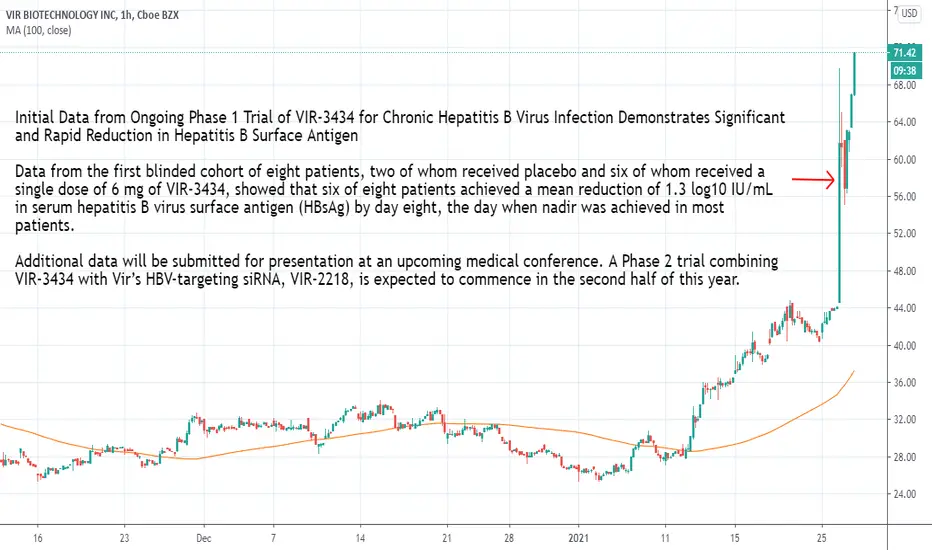
Initial Data from Ongoing Phase 1 Trial of VIR-3434 for Chronic Hepatitis B Virus Infection Demonstrates Significant and Rapid Reduction in Hepatitis B Surface Antigen
Data from the first blinded cohort of eight patients, two of whom received placebo and six of whom received a single dose of 6 mg of VIR-3434, showed that six of eight patients achieved a mean reduction of 1.3 log10 IU/mL in serum hepatitis B virus surface antigen (HBsAg) by day eight, the day when nadir was achieved in most patients.
Additional data will be submitted for presentation at an upcoming medical conference. A Phase 2 trial combining VIR-3434 with Vir’s HBV-targeting siRNA, VIR-2218, is expected to commence in the second half of this year.
finance.yahoo.com/news/initial-data-ongoing-phase-1-130000526.html
Data from the first blinded cohort of eight patients, two of whom received placebo and six of whom received a single dose of 6 mg of VIR-3434, showed that six of eight patients achieved a mean reduction of 1.3 log10 IU/mL in serum hepatitis B virus surface antigen (HBsAg) by day eight, the day when nadir was achieved in most patients.
Additional data will be submitted for presentation at an upcoming medical conference. A Phase 2 trial combining VIR-3434 with Vir’s HBV-targeting siRNA, VIR-2218, is expected to commence in the second half of this year.
finance.yahoo.com/news/initial-data-ongoing-phase-1-130000526.html
免责声明
这些信息和出版物并非旨在提供,也不构成TradingView提供或认可的任何形式的财务、投资、交易或其他类型的建议或推荐。请阅读使用条款了解更多信息。
免责声明
这些信息和出版物并非旨在提供,也不构成TradingView提供或认可的任何形式的财务、投资、交易或其他类型的建议或推荐。请阅读使用条款了解更多信息。